Nitric Acid
HNO₃ Properties, Uses, Prices, Videos, Concentrations, Grades, Safety (SDS)
Buy nitric acid solutions online or order by phone. Watch videos and review product properties, uses, prices, HNO₃ concentrations, grades and safety info.
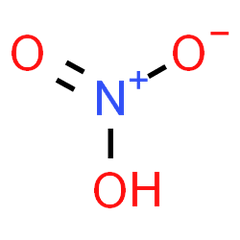
Nitric Acid is a colorless liquid inorganic acid with formula (HNO3) composed of the interaction of the nitrogen atom with the hydroxyl group and oxygen. The bond of these atoms results in the creation of this highly corrosive chemical. Its pure composition is colorless but, over time, tends to acquire a yellowish appearance due to the oxidation of the Nitrogen.
Availability
Nitric Acid is available in all sizes ranging from 500ml to a pallet for quick shipping to your facility.
Industries That Use Nitric Acid
Common Uses and Applications for Nitric Acid
- Fertilizers
- Explosives
- Dye Intermediate
- Gold Recovery
- Engraving
- Purifying Metals
- Laboratory Chemical
Filters




















