Ethanol Ethyl Alcohol
Various grades and proofs of undenatured and denatured ethanol
Shop Lab Grade Ethanol Products
We offer a diverse portfolio of high quality organic ethanol, denatured ethyl alcohol, organic pure sugarcane ethanol, and lab grade ethanol at extremely competitive prices. We are constantly adding various grades and proofs of undenatured and denatured ethanol to meet our customer's needs.
Welcome to our extensive collection of ethanol products, where quality meets affordability. Our range boasts a diverse selection, including high-quality organic ethanol, denatured ethyl alcohol, pure sugarcane ethanol, and lab-grade ethanol, all offered at exceptionally competitive prices.
At Lab Alley, we pride ourselves on continuously expanding our inventory to cater to the diverse needs of our customers, with various grades and proofs of both undenatured and denatured ethanol readily available.
On-budget and on-time, every time.
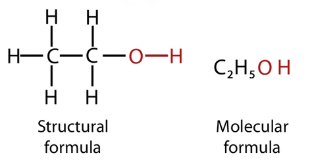
Ethanol Formula
What Makes Ethanol Essential?
What is it about this molecule that makes it so useful, and in many ways essential? Ethanol has a polar end (-OH), that looks and behaves like water, but also a carbon chain that gives ethanol nonpolar characteristics. Because that carbon chain is not very long (only 2 carbons), ethanol is still miscible with water, and can also dissolve a wide range of molecules – both slightly polar and slightly nonpolar.
Hence it is an effective solvent for many flavor compounds, coloring agents, and bioactive or medicinal compounds. By the same principle, ethanol is able to disrupt intramolecular hydrogen bonding in viruses in order to denature the protein structure and render the virus inactive. As a fuel, ethanol can produce energy during an exothermic combustion reaction. As an imbibement and food additive, ethanol is the only aliphatic alcohol that is relatively safe to consume since it can be metabolized by the human body.
Such a range of applications necessitates an equally diverse range of grades and proofs. For example, absolute ethanol (pure, 100%) is an ineffective disinfectant – it must be combined with water at 60-80% in order to denature proteins. As a fuel, crude purification of ethanol is sufficient, but as a food additive or analytical solvent, high purity is critical to the health of consumers and the integrity of the analysis.
Lab Alley provides a diverse portfolio of products to support the myriad applications.


























